Glossary
The English version of our glossary is currently under construction and will be regularly updated.
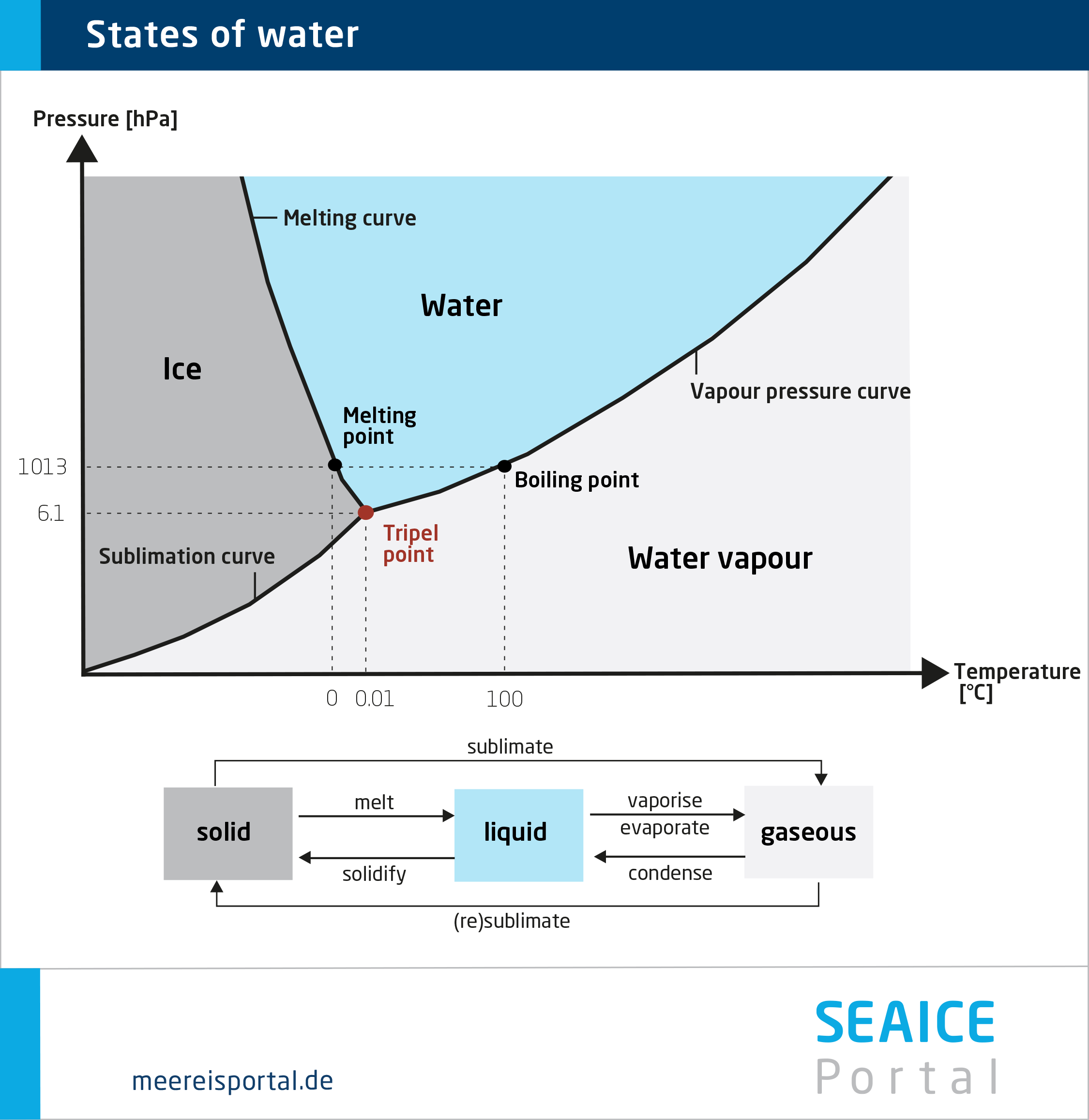
Phase transition
A phase transition is a physical process by means of which a given material changes from one state to another, i.e., changes from a solid, liquid or gaseous state to another state.
For example, at 0 degrees Celsius water changes from liquid to solid (freezes), while it changes from liquid to gas at 100 degrees Celsius (boils/evaporates).
Phase transitions can be triggered by temperature changes, pressure changes, or changes in a material’s composition. These transitions can produce unique physical characteristics, like the density anomaly of water at the transition from liquid to solid (negative thermal expansion).