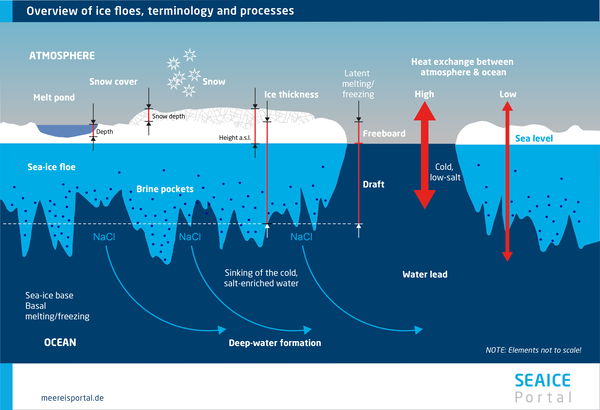
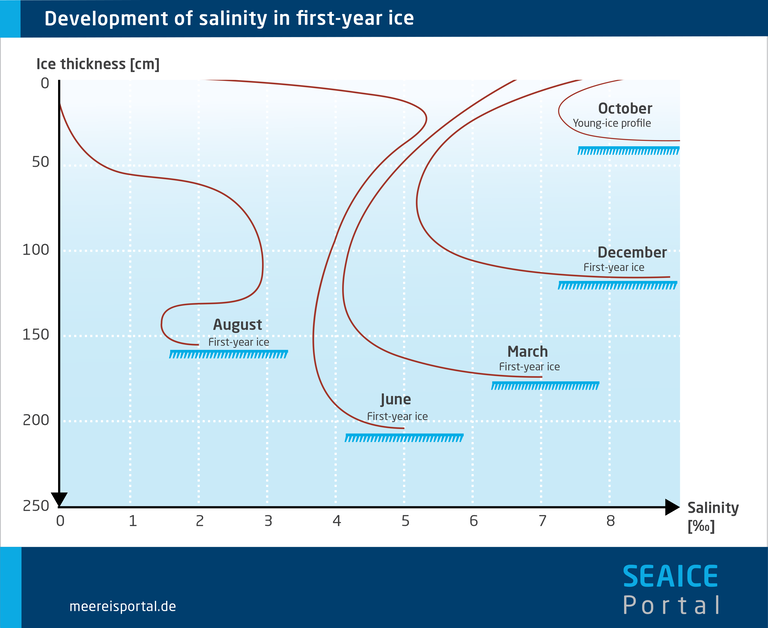
Sea-ice Desalination
Sea ice does not have a uniform salinity through all layers. In fact, as a rule it has a distinctive salinity profile, and its salinity constantly changes with increasing layer depth. This is largely due to two mechanisms that lead to desalination:
- Processes that are activated in “cold” ice, i.e., during the ice’s growth phase. These are mainly driven by temperature gradients in the existing ice cover and the cooling of individual ice layers.
- Processes that chiefly take place in “warm” ice. These require the presence of low-saline meltwater on either the surface or underside of the ice.
The first group of processes includes gravity drainage and brine expulsion. The most important process in the second group is flushing. Taken together, these processes are responsible for the typical “c” shaped salinity profile of first-year ice.
When seawater freezes, due to the crystalline structure that is formed, no molecules or ions – and therefore, no salts – are bound in the ice. This leads to a two-phase separation. The crystalline structure of solid ice is composed almost entirely of water molecules. In contrast, the salt contained in seawater is “trapped” in tiny channels and chambers in sea ice, in the form of liquid brine. Here, the rule of thumb is: the colder the ice becomes, the more salt is expelled, and the more concentrated the brine becomes (Hempel, 2006).
Gravity drainage is a desalination process in which gravity expels the brine from the ice into the seawater below. When the ice grows and its thickness increases, the surface gradually rises above the waterline to remain in a state of isostatic equilibrium. This produces hydrostatic pressure within the pore system, pushing the brine downward and out.
This desalination process is intensified by the negative temperature gradients in the ice itself in winter. The cold and therefore denser brine on the ice’s surface lies atop warmer and therefore lighter brine in the lower part of the ice. Within the brine channels, this produces a convection that forces the denser brine downward and out of the ice. The process culminates in a desalination of the ice and formation of a drainage system.
As a result of brine drainage, over time the ice’s overall salinity drops from an initial level of more than 10 parts per thousand to less than 5 parts per thousand. Due to ongoing desalination, older, multiyear ice has a lower salinity (3 to 3.5‰ lower) than younger, first-year ice (5‰). However, the vertical distribution of the salinity is not uniform. In multiyear ice, it is higher at the bottom; in first-year ice, it is high near the surface (8 to 16‰), declines in the middle, and rises again near the underside (Tucker et al., 1992).
Hempel G. & und I. Hempel I. (2006): Faszination Meeresforschung, Ein ökologisches Lesebuch, Verlag H. M. Hauschild GmbH, Bremen, 2006, p. 42
Tucker W. B., et al. (1992): Physical properties of sea ice relevant to remote sensing, in Carsey (1992): Physical properties of sea ice relevant to remote sensing, pp. 9-28
Another form of desalination in “cold” ice is brine expulsion, which only takes place in response to further cooling. Substantial “brine pockets” can form in sea ice. When they cool further, new ice forms within them. Given its larger volume, the ice creates tremendous pressure within the pockets, eventually causing them to burst and release their brine into the surrounding seawater. When this happens near the ice’s surface and the brine is expelled upward, it can form what are known as frost flowers. Measuring one to three centimetres in diameter, they are produced when the water in the expelled brine evaporates. This process leaves behind high-saline crystals that rapidly melt again.
Flushing refers to a specific form of gravity drainage. When large amounts of heavy meltwater gather on the ice’s surface in late spring and summer, it exerts hydrostatic pressure on the pore system below. The meltwater seeps into the ice, displacing the high-saline brine. Flushing is the most effective desalination mechanism and causes the ice to rapidly lose salinity in the summer months.