Sea Ice as Habitat
The continents ‘wander’. Over the course of millions of years, they change their position by hundreds or even thousands of kilometres. This continental drift is what led to the formation of two unique regions at the North and South Poles that are characterised by extreme climatic conditions – the Arctic and the Antarctic. Plants and animals living here have to be specifically adapted to the harsh climate; they must be able to cope with the ice cover, low temperatures and widely fluctuating salt levels. On top of this comes the change from dark to light. During the Polar Night the sun doesn’t shine at all; in summer, it never stops. This means that the algae living here are subjected to considerable fluctuations in the available light. Since they can neither grow nor reproduce in the dark, the production of biomass, so-called primary production, here is significantly lower than in other ocean regions in dark periods. As such, organisms that feed on algae have to be able to get by with a food supply that is severely limited at times.
We know that there are a vast number of species in the Arctic and Southern Oceans. However, the precise number is still unknown, since large parts of the polar deep sea are yet to be investigated, and comprehensive species counts are difficult to conduct. The inhabitants of the Arctic and Antarctic include minute organisms like bacteria, archaea and microalgae; invertebrates; fishes; seals and whales. Here they occupy different niches – some species live on the sea ice, some at its edges, and others beneath or within it.
Generally, experts describe the organisms that live in the polar regions as cold-adapted. They can survive here because their metabolisms are optimally adapted to the conditions. For example, some organisms – especially microorganisms – are able to rapidly start growing once winter is over and it gradually becomes warmer. As a result, the ecosystem quickly comes to life again when the temperature increases even slightly after the winter. This rapid metabolic ‘kickstart’ can be explained by the fact that the assimilation of organic and inorganic substances – and therefore also of nutrients – is temperature-dependent. According to what is known as the reaction-rate temperature rule, a temperature rise of 10 degrees Celsius increases the rate of chemical reactions two- to three-fold.
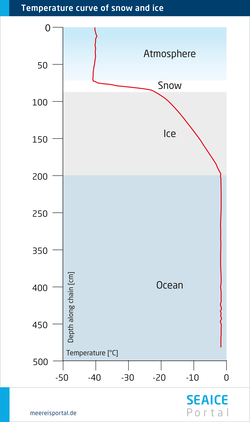
However, the sea-ice mass doesn’t all warm uniformly. Accordingly, there can be different temperatures within an ice layer, whereby the temperature change is more or less linear from top to bottom. In a simple case, there is a temperature equilibrium at the interface between the atmosphere and ice, as well as that between the ice and seawater (Arrigo, 2014). In winter, the temperatures are lower at the surface of the ice, since the atmosphere is significantly colder than the sea. The ice has an insulating effect on the underlying seawater. Ultimately, the ice decreases the heat exchange between the seawater and atmosphere. Snow cover on the ice has an additional insulating effect and can further reduce heat exchange with the atmosphere (see Figure 1).
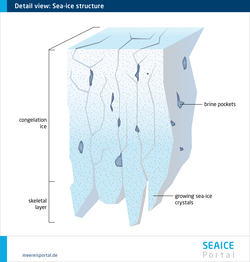
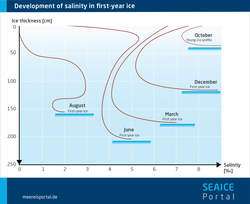
Another important environmental parameter that influences life in the ice is the salt content, or salinity. What is key here is that the salinity of the ice differs from that of normal seawater, and can be significantly higher. Higher salinity can mean that the algae that normally live in the ice are temporarily unable to survive there. The reason for this can be found in the fine structure of the ice. When seawater freezes, a large portion of the dissolved salt it contains isn’t incorporated in the crystal structure of the sea ice. Instead, it is expelled and sinks to the water layers below the ice. The older the ice, the more salt is released – in other words, the less saline it becomes. The remaining salt is then found in the ice in the form of highly concentrated saltwater, known as brine. Due to its high salinity, brine doesn’t freeze, but instead forms channels and pockets. These are partly responsible for making the ice porous (see Figure 2). Based on the structure of the brine channels and the volume of brine, experts can determine various age categories for ice. Within the ice, there are areas with varying levels of salinity – the actual ice, which hardly contains any salt, and the brine channels. If you look at the entire body of ice from its surface to its underside, multiyear sea ice, which has had the salt ‘drained’ out if it, has an average salinity of 1, while seasonal ice has a salinity of 5 to 8 (see Figure 3). The snow layer also plays a role in the low salinity of the multiyear ice: when the snow melts, the meltwater reduces the ice’s salinity. For comparison: seawater has a salinity of ca. 35.
Regardless of this aspect, the salinity fluctuates over the course of the year. In winter, when temperatures are low and the ice is compact, the salt level in the brine pockets is extremely high (up to 150). But in spring, when the sea ice melts, and in autumn, when it forms and there is exchange with the seawater, its salinity is similar to that of seawater (Arrigo, 2014). The algae are so flexible that, as a rule, they can cope with theses fluctuations, tolerating salinities of between 15 and 30 during the melting period, and even higher salinities in winter.
The crucial factor when it comes to algal growth and reproduction, however, is light. Like terrestrial plants, algae also use photosynthesis, a process by means of which they absorb carbon dioxide from the atmosphere and convert it into biomass that contains large amounts of energy-rich sugar. The ice reduces the amount of light that reaches the ocean, especially when it is thick, multiyear ice or when it is covered by a layer of snow. The reflection of sunlight plays a major role here. The measure of a surface’s ability to reflect the sun’s rays is called albedo. Measurements have shown that a snow-covered ice layer can have an albedo of more than 80 percent. If the snow is wet, it reflects fewer of the sun’s rays, since it is darker and therefore absorbs more of the light. Under such snow cover, light can only penetrate roughly a metre into the ice, but even if the ice is free of snow, light can’t penetrate deeply. Like shade-tolerant plants, the photosynthesising algae in the sea ice are adapted to these conditions. They are able to survive with little light and start to photosynthesise early in the year, when it’s still too dark for other marine algae (Arrigo, 2014).
Even more extreme are the light conditions at greater ocean depths in Arctic and Antarctic waters. Almost no light reaches the seafloor, which means that the organisms living there, the benthos, are unable to photosynthesise. One exception: shallow coastal waters, where some sunlight penetrates down to the seafloor (Piepenburg & Gutt 2014). Added to the lack of light are the low temperatures and the shortage of food on the seafloor . Therefore, benthic life forms in the polar regions are heavily dependent on the organic material that sinks to the seafloor from the ocean’s surface and the ice. How much material reaches them depends on two factors: on the one hand, the amount of biomass that the ice algae and free-floating sea algae produce, and on the other, to what extent the algal biomass is processed in the water. Algae are eaten by zooplankton, which excrete faeces that sink to the seafloor. Dead zooplankton and algae also gradually sink to the bottom. However, some of the sinking faeces and plankton remains are processed by microorganisms, and as such only a portion of the original biomass produced by algae reaches the seafloor.
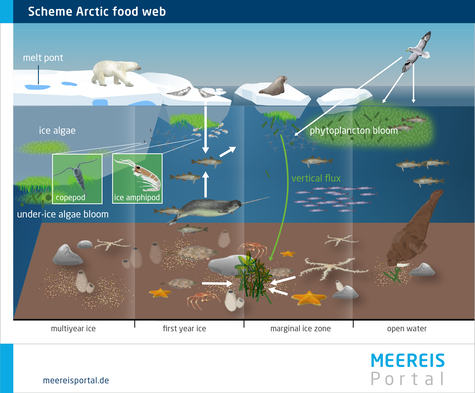
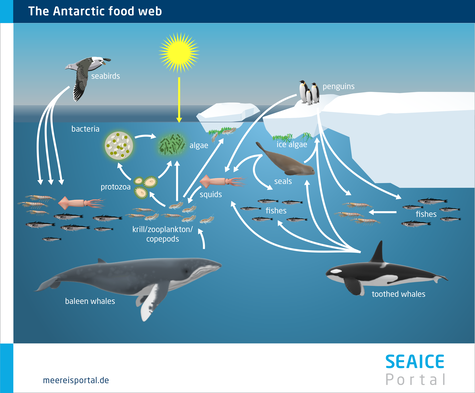
All living organisms in the Arctic and Antarctic ecosystems are interconnected by a complex food web (Figures 6 and 7). There are numerous interactions that, in many cases, make organisms highly dependent on one another. The food web consists of various levels, known as trophic levels. Organisms with similar feeding habits are classified in the same trophic level – for instance those animals that feed on algae. Animals in higher trophic levels feed on organisms in the levels lower down. To explain the complex processes in the food web, in the following, the main trophic levels are described using a simplified food chain. Within this food chain, energy and materials flow from the primary producers and photosynthesising organisms (lowest trophic level) to the tertiary consumers, like polar bears and seals (highest trophic level). The food chain consists of level 1, the primary producers; level 2, the primary consumers; and levels 3 and 4, the secondary and tertiary consumers. Primary consumers, e.g. zooplankton, feed on algae, while secondary and tertiary consumers feed on organisms from levels 2 and 3. Every time there is a transition to the next level, some energy is lost, since all organisms use energy – e.g. to move, to reproduce, or to produce body heat. As a result, with every transition to the next trophic level, ca. 10 percent of the available food is used to increase body mass (Nentwig et al., 2011). In a food chain with primary producers and three trophic levels above them, theoretically only 0.1 percent of the primary production is available for the final, highest trophic level.
In reality, however, there are numerous intermediate levels. For instance, copepods can feed on algae as well as animal food sources, and accordingly can be categorised in two trophic levels. Therefore, experts often use detailed food webs in order to provide a more realistic depiction of the relationships between the various species. Along with the producers and consumers, the food web also includes the destruents (decomposers), particularly microorganisms, which utilise organic material from all trophic levels. During the decomposition process, organic matter is remineralised and released as inorganic food sources. These, in turn, are taken up by the primary producers, which produce organic substances through photosynthesis, thereby completing the cycle.
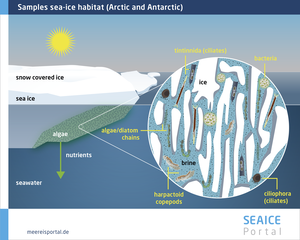
Many people associate life in the Arctic and Antarctic with typical species like polar bears, penguins, seals and whales. However, life at the microscopic scale is also incredibly diverse. There are a broad range of organisms, including algae and microorganisms, as well as nematodes. These organisms populate the brine channels, which often leads to large accumulations of these communities at the surface and in the boundary layer between the ice and water (Figure 4), since the water provides vital nutrients. As a result, at the boundary between the sea ice and seawater, for instance, brine seepage and ice growth produce a convection current that carries nutrient-rich water to the ice. A further important habitat is the land-based platelet ice, which forms beneath the sea ice at the edges of the Antarctic ice shelf region. Being thin and porous, it allows significant exchange with the nutrient-rich seawater. Especially in the upper layers of the platelet ice there is a high concentration of algae, since this is where the most light can be found (Arrigo, 2014). Algal growth on the underside of the ice can also be considerable. The Arctic diatom species Melosira arctica (Figure 8) and the Antarctic alga Berkeleya antarctica frequently occur as communities: chains of algae that grow downward beneath the ice like giant threads. The communities provide large quantities of biomass and can reach several metres in length. When the ice melts, the algae lose their hold and sink to the seafloor, which means that they are also an important food source for benthic organisms (Boetius et al., 2013).
Diatoms are a vital source of food for many other organisms. Some diatoms enter the seawater with brine from the ice. Large numbers of algae are released in spring, when the ice melts. Since the meltwater contains less salt than the surrounding seawater and has a lower density, it forms a stable surface layer in which the algae accumulate. Because they are near the water’s surface, they have optimal light conditions. At the edges of the large sea-ice masses, the light conditions are particularly good, as a result of which massive algal blooms form. Primary consumers – like amphipods and copepods or fish larvae, which belong to the category zooplankton – benefit from this. With the algal blooms, the populations of zooplankton automatically increase, which in turn attracts animals from the next trophic level, the secondary consumers, which include larger fishes and birds. The large fishes are then eaten by tertiary consumers such as seals, narwhals and polar bears, which are the final component in the food web.
Comparatively few algae are found within the sea ice. Particularly in winter, the internal ice structures are characterised by the very low temperatures and very high salinity in the brine channels, since the volume of brine is low and its salinity extremely high – unfavourable conditions for algae to survive. With the spring melt, on the other hand, the volume of brine increases again and the salinity in the brine channels decreases, since there is exchange with the seawater below. At the same time nutrient transfer occurs once again, which allows the algae to grow. There is also increased nutrient exchange in the ice cracks and at the ice edge, promoting algal growth here, too (Arrigo, 2014).
Algal communities are also found in meltwater pools. These pools form mainly on Arctic sea ice as a result of the snow on top melting. Melted snow reduces the albedo, creating depressions in which water collects. However, since the meltwater pools contain few nutrients, the amount of algal biomass is generally low. Due to the increasing temperatures accompanying global warming, large meltwater pools now form in the Arctic in summer, and during the warm months, these can become so deep that the meltwater comes into contact with the seawater and nutrient transfer takes place. As a result, in such cases the amount of microbiological biomass in the pools is ultimately greater than in the water column below them. Although, as a rule, the microbiological activity in meltwater pools is lower than in other marine habitats, they nonetheless offer an important source of food for multicellular organisms (Arrigo, 2014).


AMAP (2011): Snow, Water, Ice and Permafrost in the Arctic (SWIPA): Climate Change and the Cryosphere. Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, xii + 538 pp; figure 9.29.
Arrigo, K. R. (2014): Sea ice ecosystems, Ann. Rev. Mar. Sci., 6, 439–67, DOI: 10.1146/annurev-marine-010213-13510. The Annual Review of Marine Science is online at marine.annualreviews.org
Boetius, A. et al. (2013): Export of Algal Biomass from the Melting Arctic Sea IceScience 339 (6126), 1430-1432. DOI: 10.1126/science.1231346.
CAFF (2013): Life linked to ice. Conservation of Arctic Flora and Fauna, Akureyri, Iceland.
Campbell, N. A., L. A. Urry, M. L. Cain, S. A. Wasserman, & P. V. Minorsky (2017): The Ecology of Life. In: Author Biology: A Global Approach, Global Edition (English Edition) 11. Auflage, p. 1280, Chapter: The Ecology of Life; Figure: 54.15 An Antarctic marine food web.
Griffiths, H.J. (2010): Antarctic Marine Biodiversity – What Do We Know About the Distribution of Life in the Southern Ocean? PLoS ONE 5(8): e11683. doi.org/10.1371/journal.pone.0011683
Nentwig, W., M. Bacher & R. Brandl (2011): Ökologie kompakt, Springer Spektrum, 3. Auflage, pp. 114-122
Petrich C., H. Eicken in D. N. Thomas & G. S. Dieckmann (2017): Sea Ice an introduction to ice physics, chemistry, biology and geology, Blackwell Publishing, p. 12
Piepenburg, D. & J. Gutt (2014): Klimabedingte ökologische Veränderungen in den Bodenfaunen polarer Schelfmeere. In: J. L. Lozán, H. Graßl, D. Notz & D. Piepenburg (Eds.), Warnsignal Klima: Die Polarregionen, pp. 152 – 159.